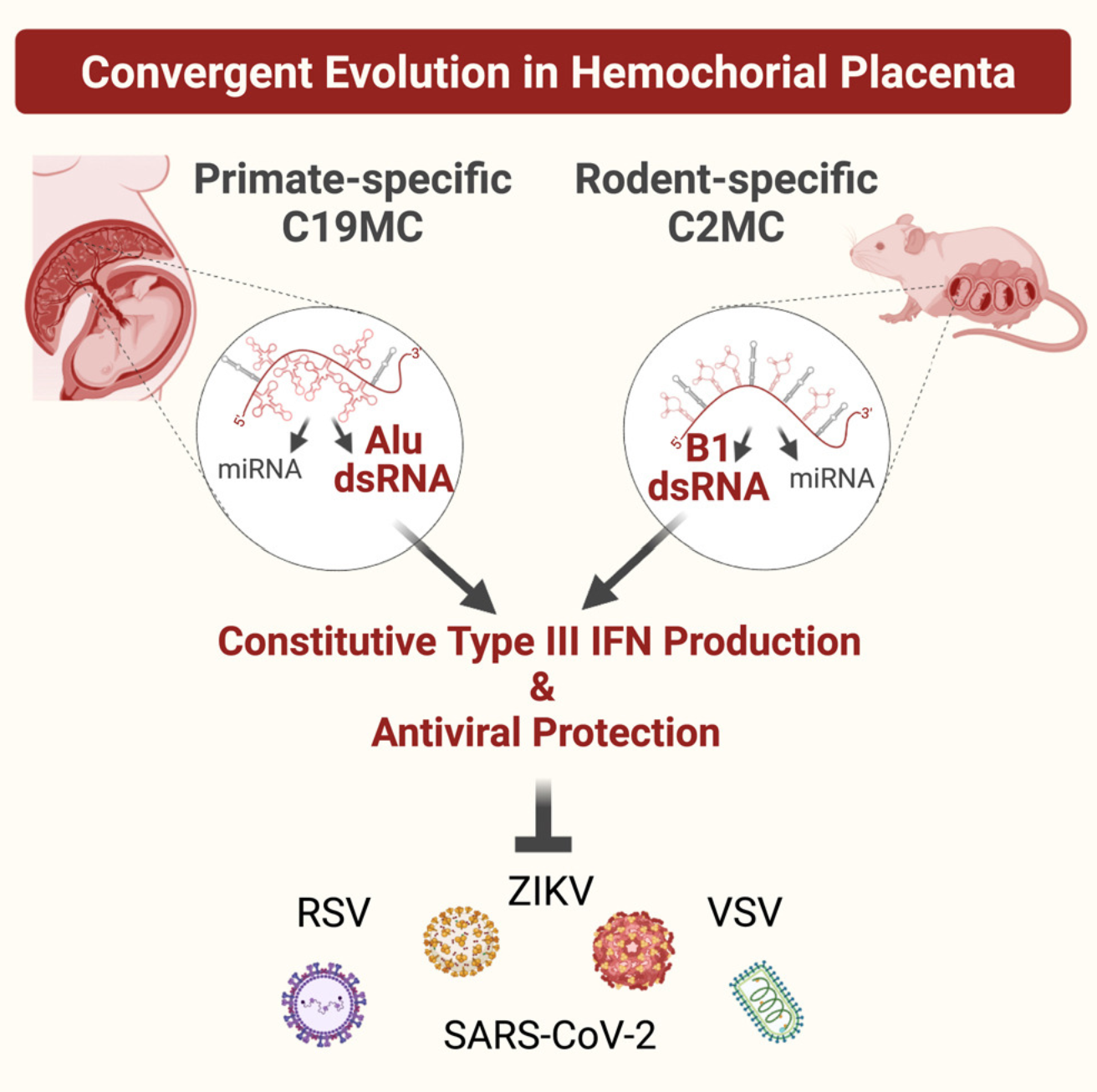
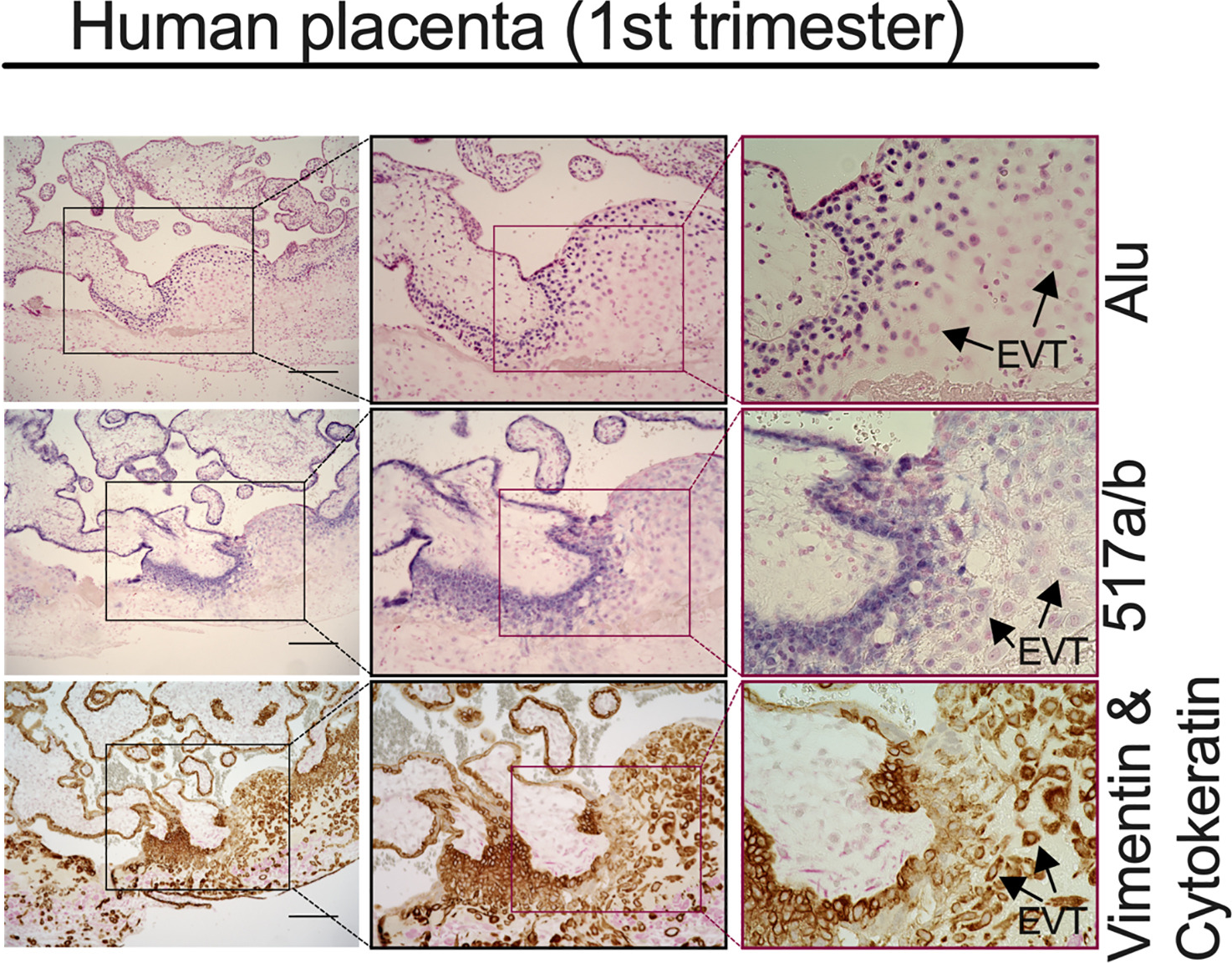
Hemochorial placentas have evolved defense mechanisms to prevent the vertical transmission of viruses to the immunologically underdeveloped fetus. Unlike somatic cells that require pathogen-associated molecular patterns to stimulate interferon production, placental trophoblasts constitutively produce type III interferons (IFNL) through an unknown mechanism. We demonstrate that transcripts of short interspersed nuclear elements (SINEs) embedded in miRNA clusters within the placenta trigger a viral mimicry response that induces IFNL and confers antiviral protection. Alu SINEs within primate-specific chromosome 19 (C19MC) and B1 SINEs within rodent-specific microRNA cluster on chromosome 2 (C2MC) produce dsRNAs that activate RIG-I-like receptors (RLRs) and downstream IFNL production. Homozygous C2MC knockout mouse trophoblast stem (mTS) cells and placentas lose intrinsic IFN expression and antiviral protection, whereas B1 RNA overexpression restores C2MCΔ/Δ mTS cell viral resistance. Our results uncover a convergently evolved mechanism whereby SINE RNAs drive antiviral resistance in hemochorial placentas, placing SINEs as integral players in innate immunity.
Atherosclerotic Cardiovascular Disease

injured arteries treated with cell selective
Ad-p27-126Ts miRNA switch

exhibiting restenosis after balloon injury