Research

Research Focus:
Channels and Transporters in Renal and Vascular Function
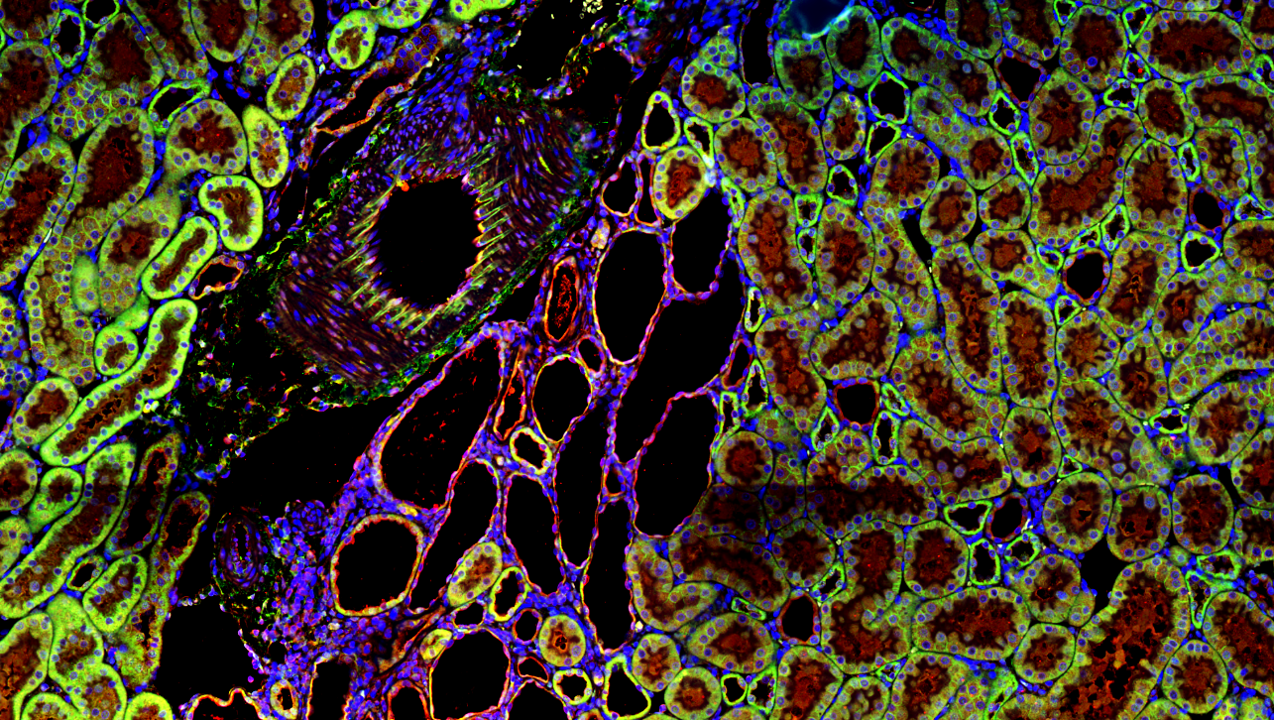
Taurine Transport in Polycystic Kidney Disease
Polycystic kidney disease (PKD) is predominantly caused by mutations in PKD1, PKD2, and PKHD1, which result in large fluid-filled cysts that damage surrounding tubules leading to end-stage renal disease. Identifying critical factors influencing cyst growth in Polycystic Kidney Disease (PKD) is essential knowledge that will lead to clinical interventions to prevent or slow the progression of this devastating disease. Preliminary data obtained with an Autosomal-Recessive Polycystic Kidney Disease (ARPKD) rat model, the PCK rat, which has a spontaneously occurring mutation in Pkdh1, determined that the cystic fluid in this model has a high concentration of Taurine. Taurine is a β-amino acid with a broad range of physiological functions, including acting as an osmolyte to preserve cell volume in hypertonic conditions. Ongoing studies in the lab are investigating 1) the effect of taurine supplementation on cystogenesis and renal disease in the PCK rat 2) mechanisms of taurine transport across cystic epithelia in different osmotic conditions and 3) the effect of taurine on cyst cell growth and calcium signaling.
Golgi Calcium in Vascular Smooth Muscle Cells
Intracellular channels and transporters are increasingly being appreciated for their critical roles in numerous organellar membranes, where they help maintain ionic concentration gradients essential for endomembrane function. One of the more challenging organelles to study has been the Golgi apparatus, due to its dynamic behavior, polarized organizational structure, and multifaceted functions. We are currently investigating how alterations to Golgi Ca2+ homeostasis impact vascular smooth muscle cell function. Current studies are looking at 1) How Golgi Ca2+ stores and regulator protein remodeling in hypertensive vascular smooth muscle cells impacts cellular function and 2) Whether a compound that reduces luminal Golgi Ca2+ can reduce blood pressure.
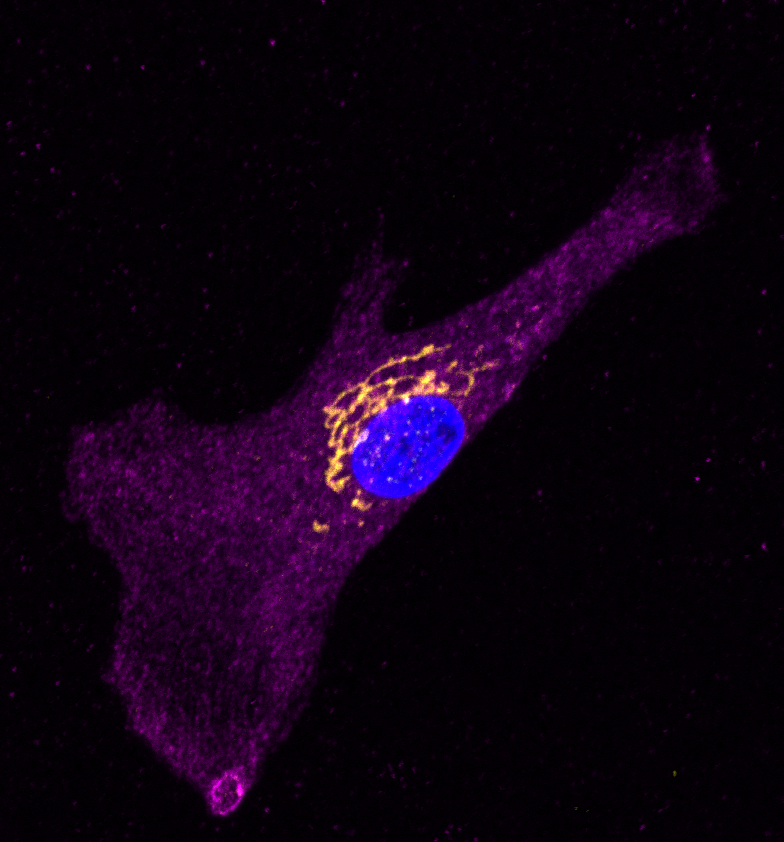

ClC-6 and Blood Pressure Regulation
Nearly half of the US adult population has hypertension, which puts them at increased risk for stroke, vascular damage, heart attack, heart failure, and kidney disease. Recent genome-wide association studies have linked a number of mutations in the gene, CLCN6, to reduced hypertension and stroke risk. CLCN6 encodes the voltage-sensitive chloride channel 6 (ClC-6). To date, very little is known of about the function of ClC-6 in blood pressure homeostasis. The focus of this project is to determine how loss-of ClC-6 function impacts vascular contractility and pathological remodeling in hypertension.

