Research
Transforming Heart Regeneration & Drug Discovery
Translational Research of Cardiomyopathy and Heart Failure
Approximately 7 million Americans over the age of 20 suffer from heart failure, which can result from ischemic heart disease, cardiomyopathy, high blood pressure, and other conditions. The prevalence of heart failure continues to rise and is expected to reach 8.5 million by 2030, primarily due to the lack of effective treatment options in clinics.
Project Goals: Understand the molecular mechanisms underlying heart failure and develop potential treatments using genetic and chemical approaches. We aim to Investigate the impact of various stresses on cardiomyocyte contractility and viability and explore the efficacy of metabolic modulators or gene therapies to restore normal cardiac function.
- Approach:
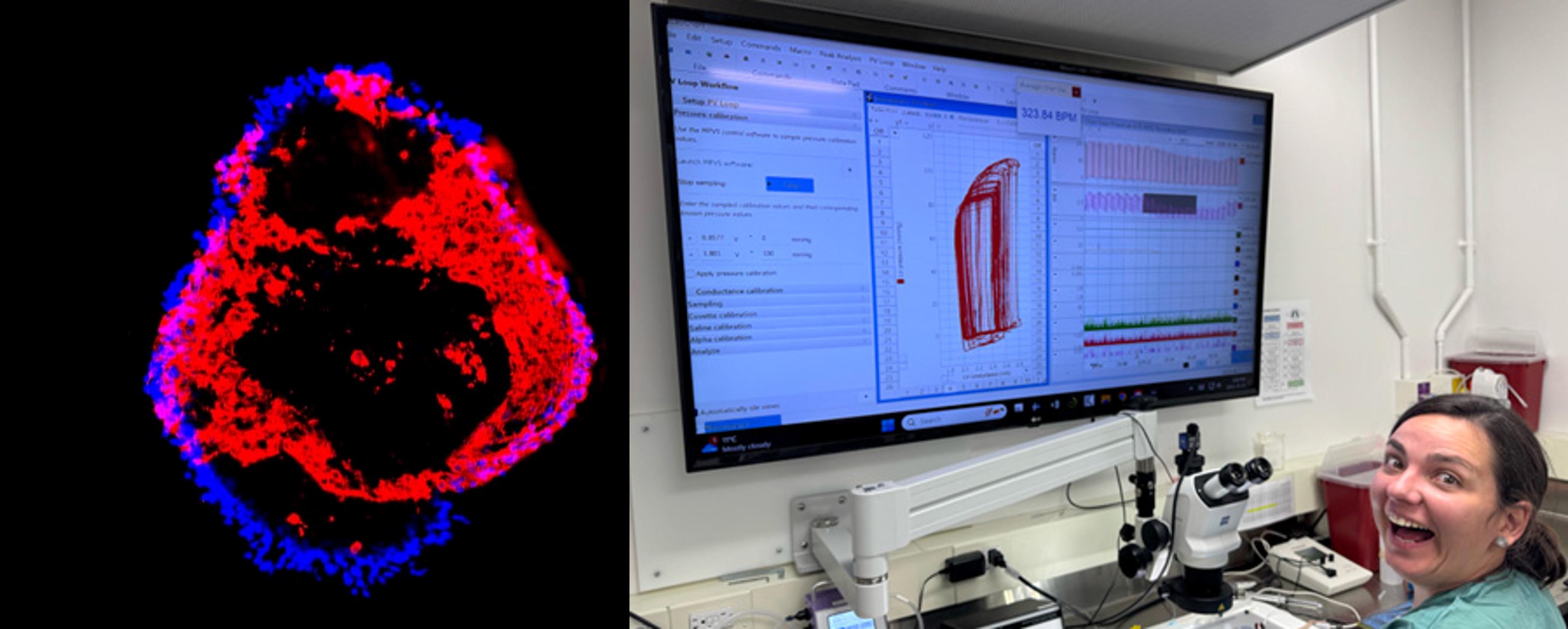
- hiPSC Models: Generate cardiomyocytes and engineered heart tissue from patients to study stress and its effects on heart cells.
- Mouse Models: Use genetically modified mice and/or surgical insults to replicate cardiomyopathy/heart failure and test interventions.
Cardiac Development and Heart Regeneration

Down syndrome (DS) caused by triplication of chromosome 21 affects 1 in 700 live births. Studies suggest that around 40-50% of people with Down syndrome are affected by some form of congenital heart defect. These can range from relatively mild issues to more severe conditions.
Project Goals: Investigate how Down syndrome (DS) affects heart development and function. DS is associated with congenital heart defects (CHDs) such as atrioventricular septal defects and ventricular septal defects. We aim to identify novel therapeutic targets or drug repurposing strategies to correct or mitigate heart defects in DS and explore the role of specific genes or gene networks (e.g., DYRK1A) in heart development and disease.
- Approach:
- hiPSC Models: Derive cardiomyocytes from DS-hiPSCs to model heart defects and study how trisomy 21 influences cardiogenesis at the cellular level.
- Mouse Models: Use mouse models with trisomy 21 or gene editing to investigate DS-specific heart defects and their molecular mechanisms.
Cardiac regeneration: Adult mammalian hearts cannot sufficiently regenerate the myocardium and restore cardiac function after stress, such as myocardial infarction. Promoting cardiomyocyte proliferation holds promise for heart regeneration. Signaling pathways that facilitate cardiomyocyte proliferation are active during heart development but become relatively inactive in the adult heart. Understanding cardiac cell fate commitment and expansion, cell positioning, and morphogenesis during heart development could provide insights into designing strategies for heart regeneration
Project Goals: Uncover the factors that determine whether cardiomyocytes proliferate, differentiate, or undergo apoptosis, and leverage this understanding for heart regeneration. We aim to identify key regulators of cardiomyocyte cell fate commitment, cell cycle progression, and survival and develop strategies to enhance the regenerative capacity of the heart by manipulating signaling pathways or gene expression.
- Approach:
- hiPSC Models: Study how different conditions or genetic modifications influence the ability of hiPSC-derived cardiomyocytes and tissues to regenerate.
- Mouse Models: Investigate the role of specific genes or signaling pathways in the repopulation of cardiomyocytes.
- Gene Editing: Use CRISPR/Cas9 or other techniques to dissect the genes' function in cell fate decisions.

