Research
Anatomical and functional mapping of sensory nerves
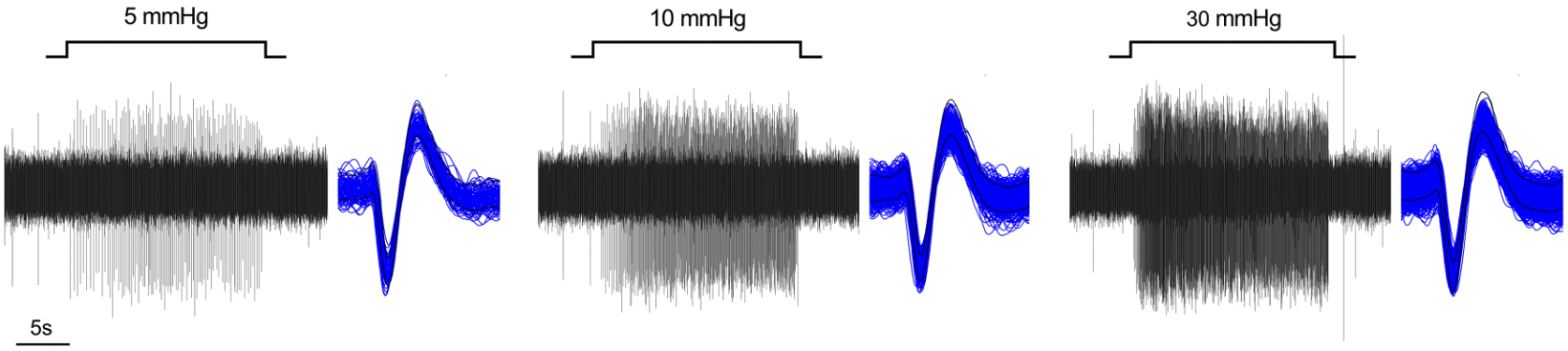
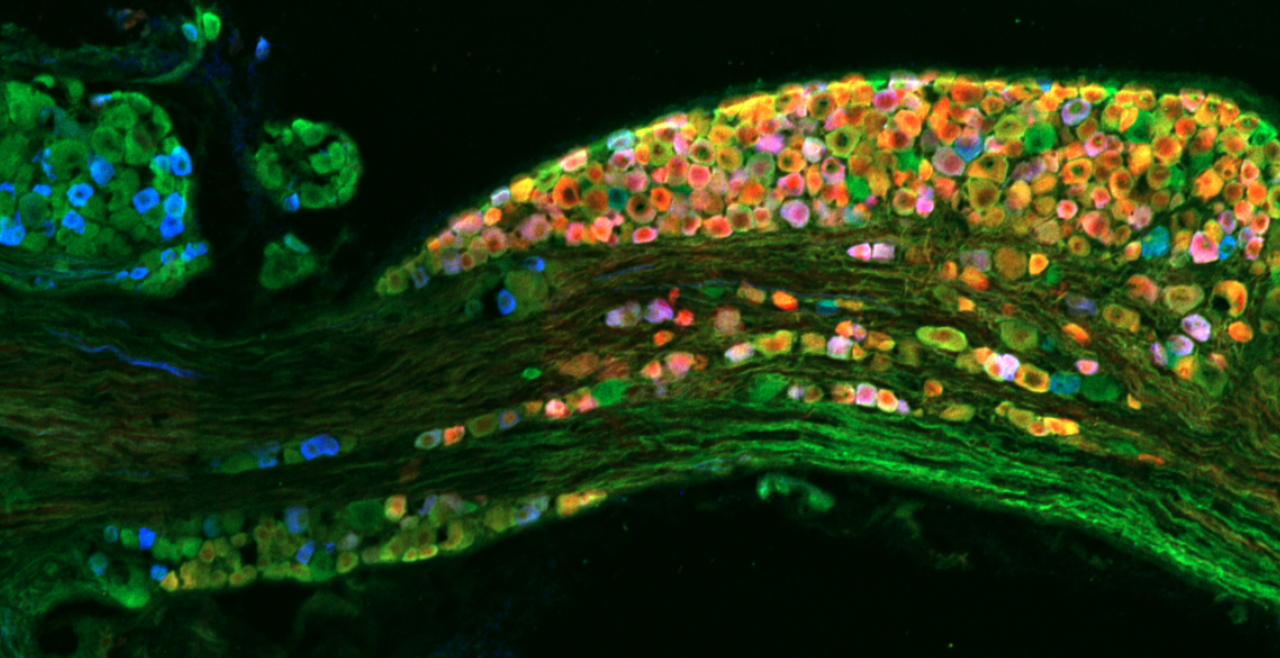
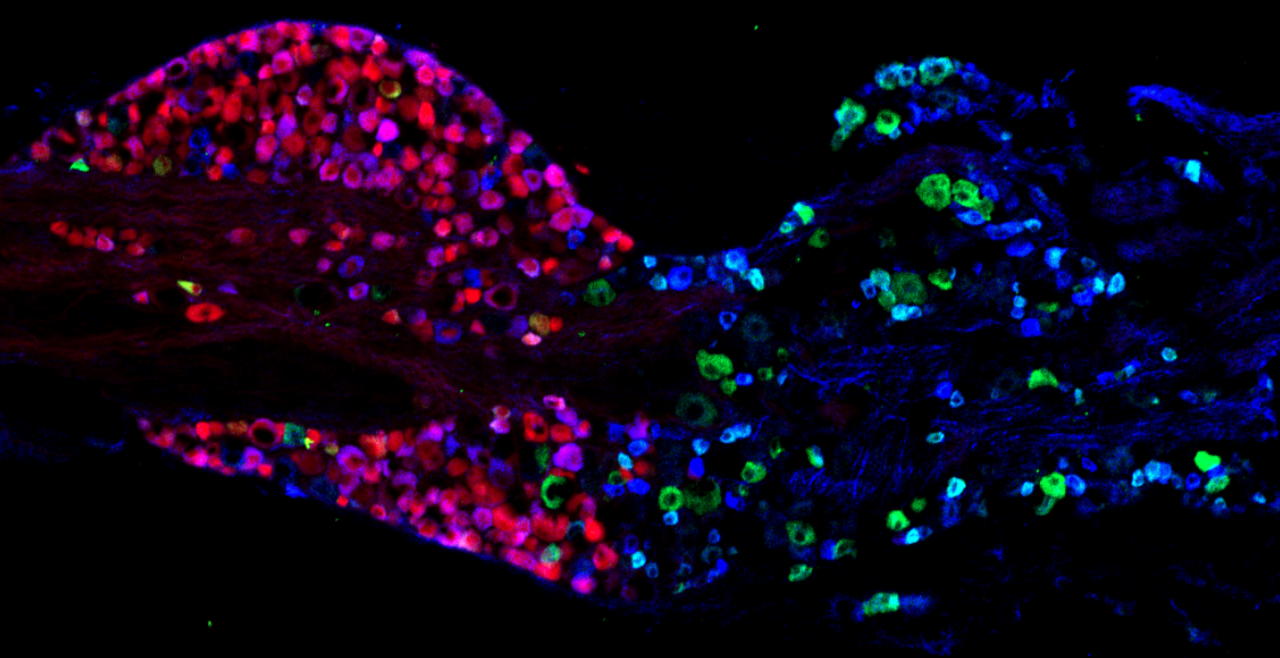
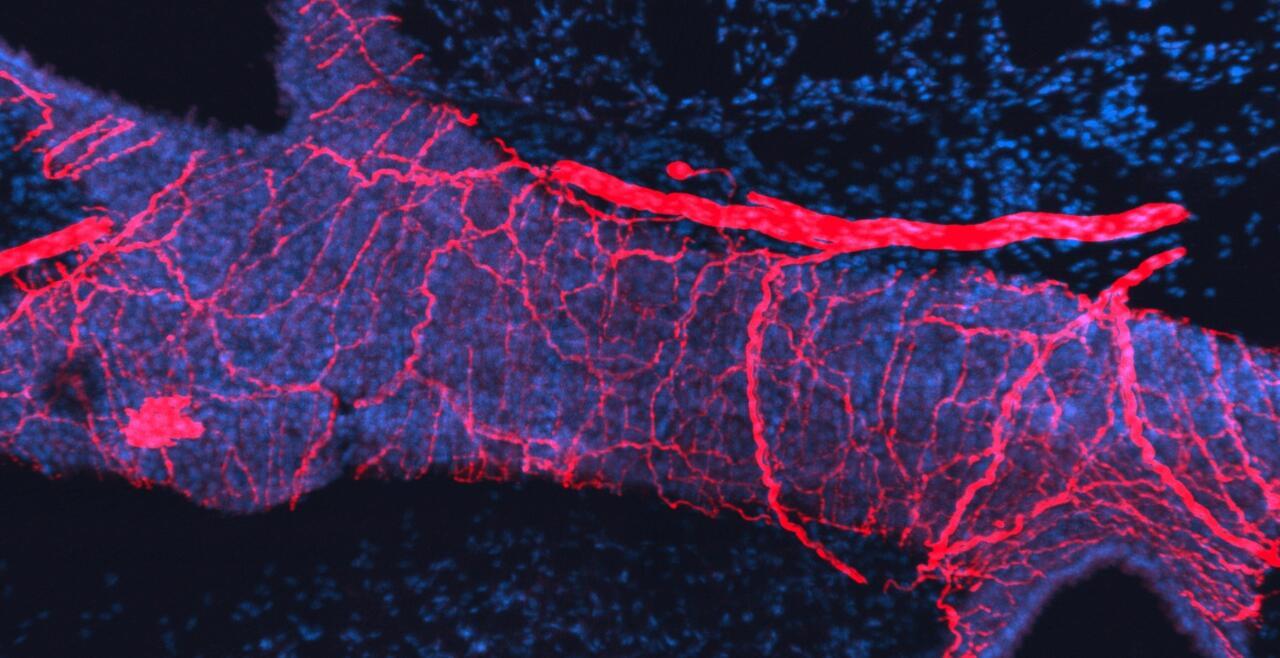
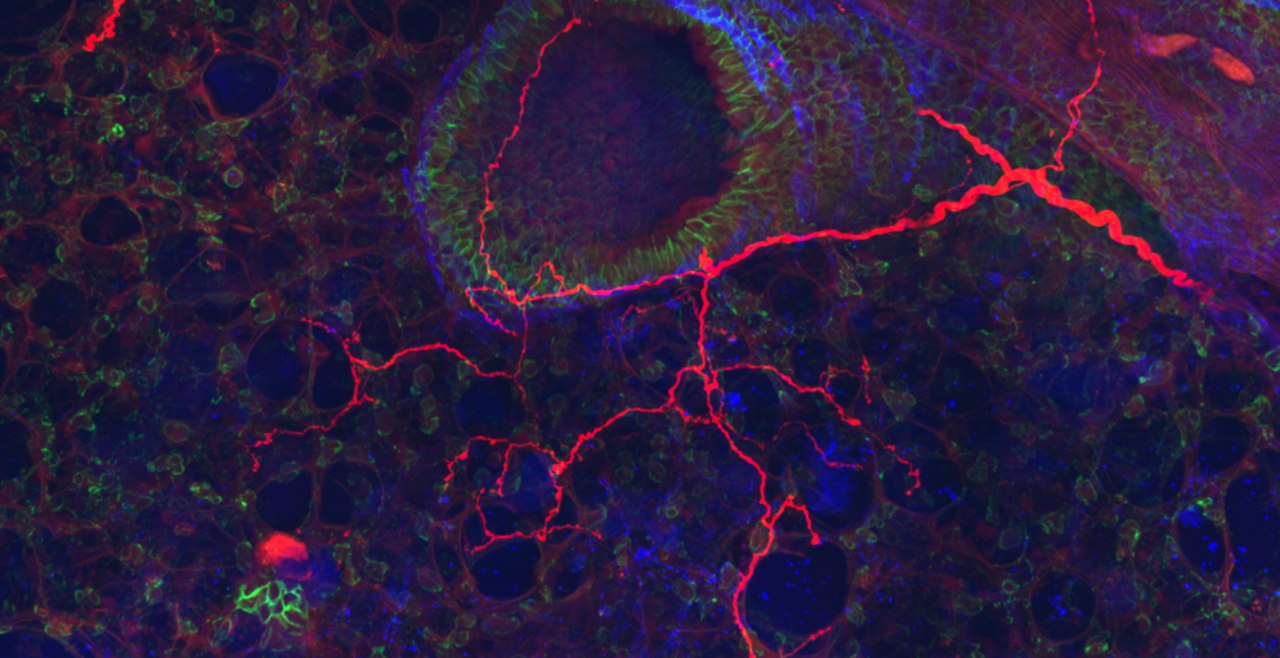
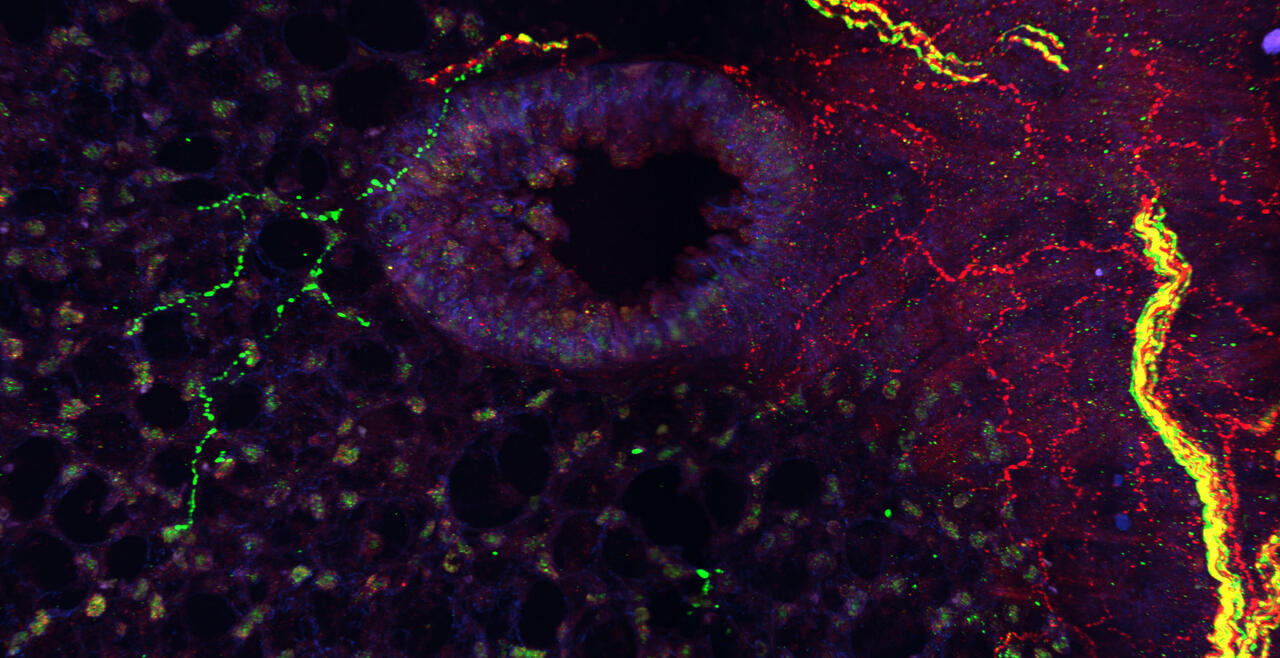
The vagal ganglion provides sensory nerve innervation to almost every organ in the thorax and abdomen. Vagal sensory nerves innervating these tissues are activated by local physiological and pathological stimuli, resulting in sensations and reflexes that serve to drive behavior and organ function that ultimately preserve homeostasis.
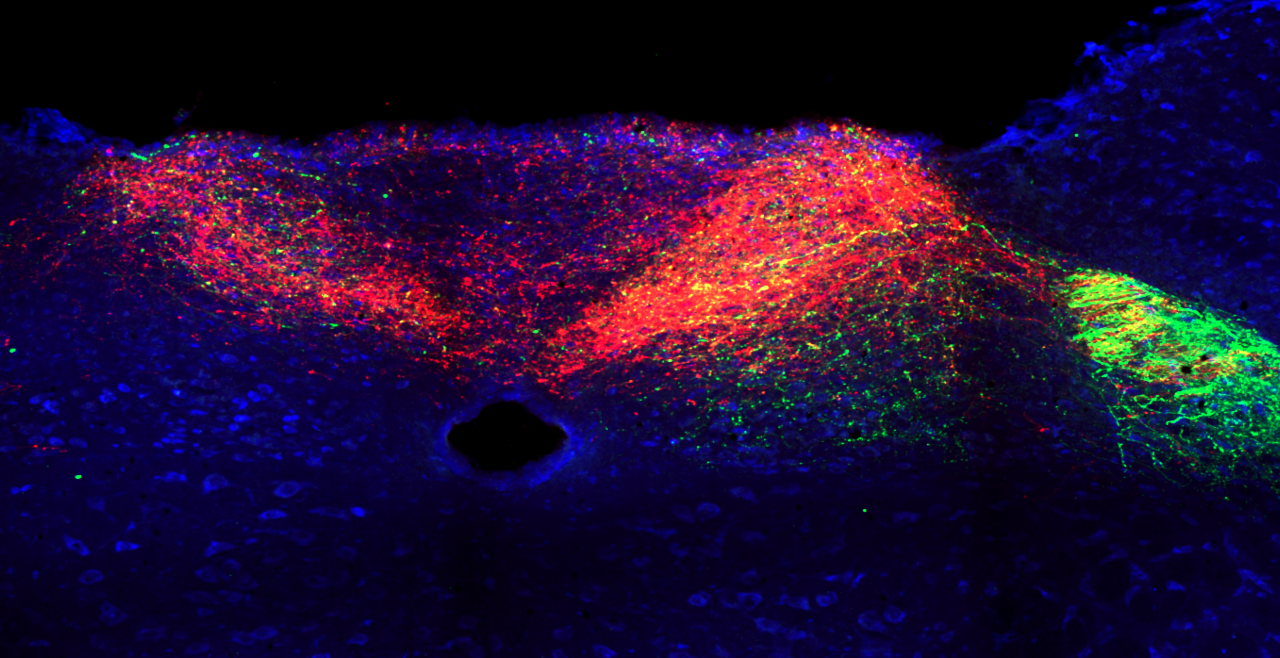
Vagal sensory nerves are heterogeneous. Some neurons are activated by noxious stimuli (e.g. acid, irritants, inflammatory mediators). These ‘nociceptive’ nerves express the ion channels TRPV1 and TRPA1 and neuropeptide neurotransmitters such as substance P or CGRP. Whereas other nerves do not express these proteins and are typically only activated by mechanical forces (‘low-threshold mechanosensors’). Lastly, the vagal ganglion is comprised of 2 regions: nodose ganglion and jugular ganglion, whose sensory neurons have distinct biochemical profiles. One of our major projects is mapping the different subsets of vagal sensory nerves, their innervation of visceral organs, their central projections, and determining their specific function in health and disease.
Irritant-evoked pulmonary-cardiac reflexes
Irritant inhalation causes reflex changes in the autonomic control of respiratory and cardiovascular function. Many irritants activate airway nociceptive sensory nerves via the gating of TRPA1. Inhalation of the TRPA1 agonist AITC causes bradycardia in normal mice and rats, via reflex increase in parasympathetic drive to the heart.
Irritant pulmonary-cardiac reflexes are remodeled in some pathological conditions. AITC inhalation in Spontaneously Hypertensive rats causes a complex mixture of bradycardia and tachycardia with premature ventricular contractions (PVCs), due to the reflex increase of both parasympathetic and sympathetic efferent nerves. Sympathoexcitation is a major risk factor for patients with pre-existing cardiovascular disease (CVD). Activation of the remodeled pulmonary-cardiac reflex is likely a cause of morbidity in CVD patients in areas with high levels of air pollution. Our group studies the mechanisms underlying the reflex remodeling, and seeks to identify therapeutic targets to reverse the remodeling.
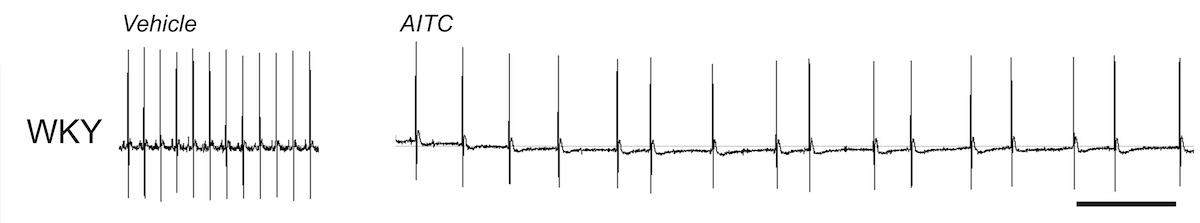
AITC evokes bradycardia in normotensive rats. ECG, scale is 1s.
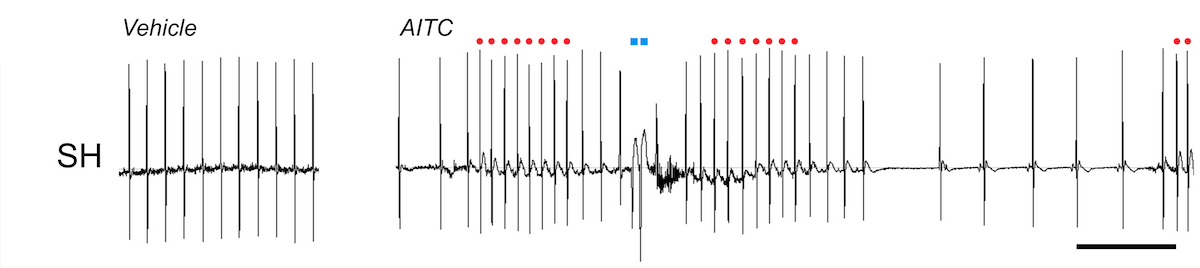
AITC evokes bradycardia with bouts of tachycardia (red) and PVCs (blue) in Spontaneously Hypertensive rats. ECG, scale is 1s.
Mechanisms of sensory nerve activation/excitability
For 20 years, we have studied the receptors, channels and pathways involved in the transduction of various stimuli into sensory nerve activity. Currently, we are focused on the contribution of different voltage-gated sodium channels in the initiation and propagation of action potentials in vagal sensory nerves.