Research

Slow-Release Drug Delivery
With our novel preparations of essential drugs in ophthalmology, we utilize unique polymer formulations and conjugation/encapsulation to develop slow-release drug release technologies.
Drug Discovery
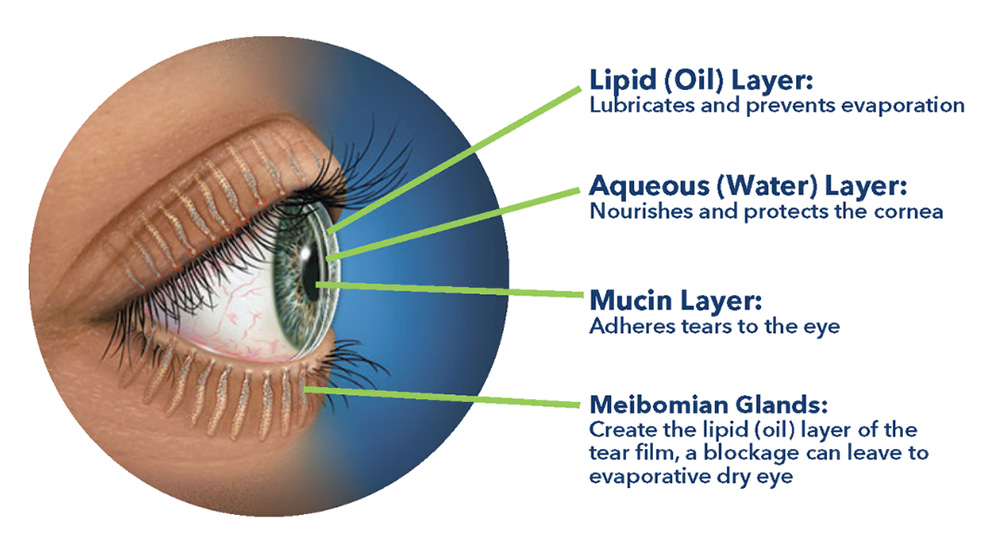
Our team is developing unique formulations for addressing dry eye disease and pan-antimicrobial solutions for all forms of keratitis (bacterial, fungal, and viral).
 Technology and Engineering
Technology and Engineering
We aim to develop innovating applications for technology and engineering, bridging it with medicine to improve patient outcomes.
