Research
The blood-brain barrier (BBB) is a dynamic structure located at the interface of the CNS and circulation system. It plays an important role in brain homeostasis and almost all neurological disorders. For example, BBB disruption occurs prior to dementia and contributes to the onset/development of Alzheimer’s disease. The BBB mainly consists of endothelial cells, pericytes, astrocytic endfeet, and a non-cellular constituent---the basal lamina. Although how various cell types affect BBB integrity is well-studied, the function of the basal lamina remains largely unknown. We aim to answer this important question and fill the gap of knowledge by targeting laminin, a major component of the basal lamina. There are five major projects in our laboratory.
Laminin and Laminin Receptors in BBB Maintenance
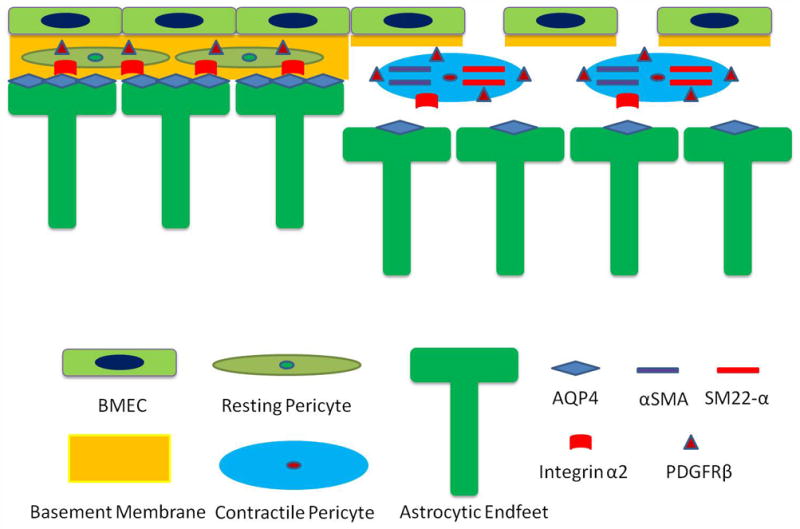 Laminin is a trimeric protein with many isoforms. Interestingly, different cell types synthesize distinct laminin isoforms. By binding to their receptors, different laminin isoforms differentially regulate BBB integrity. Specifically, astrocytic laminin actively maintains BBB integrity via regulating mural cell differentiation; endothelial laminin regulates BBB integrity mainly via transcytosis; and pericytic laminin contributes to BBB integrity in an age-dependent manner. We are currently studying the functions of non-vascular cell (microglial and oligodendrocytic) laminin as well as that of laminin receptors in BBB integrity.
Laminin is a trimeric protein with many isoforms. Interestingly, different cell types synthesize distinct laminin isoforms. By binding to their receptors, different laminin isoforms differentially regulate BBB integrity. Specifically, astrocytic laminin actively maintains BBB integrity via regulating mural cell differentiation; endothelial laminin regulates BBB integrity mainly via transcytosis; and pericytic laminin contributes to BBB integrity in an age-dependent manner. We are currently studying the functions of non-vascular cell (microglial and oligodendrocytic) laminin as well as that of laminin receptors in BBB integrity.
Laminin and Laminin Receptors in Neurological Disorders
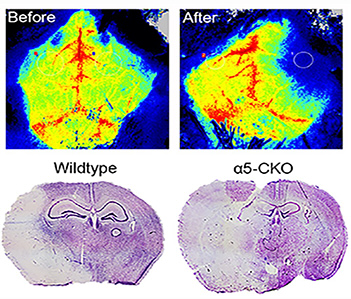 Based on the important functions of laminin and its receptors in BBB maintenance, we hypothesize that laminin signaling may affect the pathogenesis and outcomes of various neurological disorders, including stroke (both hemorrhagic and ischemic) and Alzheimer’s disease. We have recently shown that ablation of mural cell-derived laminin-γ1 or endothelial laminin-α5 aggravates hemorrhagic brain injury, while loss of mural cell-derived laminin-α5 attenuates ischemic brain injury. These findings pave the way for developing laminin-based therapies in stroke. We are currently investigating how loss of laminin affects the pathogenesis and outcomes of Alzheimer’s disease.
Based on the important functions of laminin and its receptors in BBB maintenance, we hypothesize that laminin signaling may affect the pathogenesis and outcomes of various neurological disorders, including stroke (both hemorrhagic and ischemic) and Alzheimer’s disease. We have recently shown that ablation of mural cell-derived laminin-γ1 or endothelial laminin-α5 aggravates hemorrhagic brain injury, while loss of mural cell-derived laminin-α5 attenuates ischemic brain injury. These findings pave the way for developing laminin-based therapies in stroke. We are currently investigating how loss of laminin affects the pathogenesis and outcomes of Alzheimer’s disease.
Laminin Expression and Turnover
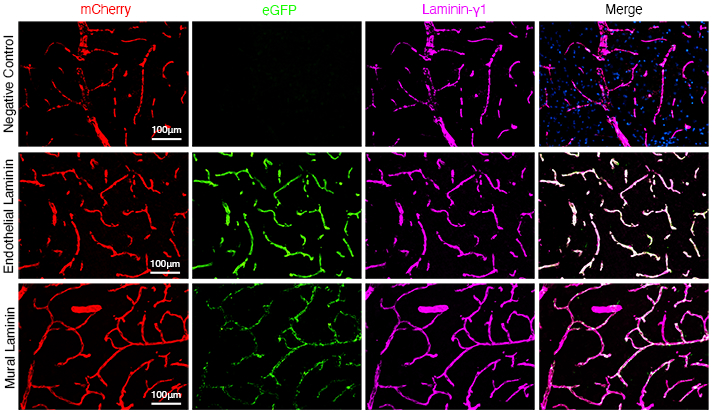 Laminin expression and turnover profiles in the CNS remain unknown. This is mainly due to the large number of laminin isoforms and the lack of research tools to accurately examine each cell type-derived laminin. We are developing innovative laminin reporter mouse lines, which allow investigation of laminin expression and turnover in a cell-specific and Cre-dependent manner. Using these genetic tools, we hope to generate a comprehensive expression/turnover profile for each cell type-derived laminin in the CNS. This information will shed light on an understudied yet extremely important field and open doors for new research.
Laminin expression and turnover profiles in the CNS remain unknown. This is mainly due to the large number of laminin isoforms and the lack of research tools to accurately examine each cell type-derived laminin. We are developing innovative laminin reporter mouse lines, which allow investigation of laminin expression and turnover in a cell-specific and Cre-dependent manner. Using these genetic tools, we hope to generate a comprehensive expression/turnover profile for each cell type-derived laminin in the CNS. This information will shed light on an understudied yet extremely important field and open doors for new research.
Pericyte and Fibroblast Biology in the CNS
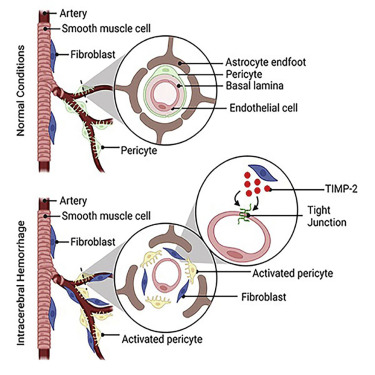 Pericytes and fibroblasts are two less studied cell populations in the CNS. Their biology and functions under physiological and pathological conditions are largely unclear. By using novel genetic models, we are able to selectively ablate these cells. We are characterizing the phenotypes of the resulting mice with or without brain injury and exploring the underlying molecular mechanisms. These findings will uncover the functional significance of pericytes and fibroblasts in brain homeostasis and neurological disorders.
Pericytes and fibroblasts are two less studied cell populations in the CNS. Their biology and functions under physiological and pathological conditions are largely unclear. By using novel genetic models, we are able to selectively ablate these cells. We are characterizing the phenotypes of the resulting mice with or without brain injury and exploring the underlying molecular mechanisms. These findings will uncover the functional significance of pericytes and fibroblasts in brain homeostasis and neurological disorders.
Adcy2 in Brain Homeostasis and Neurological Disorders
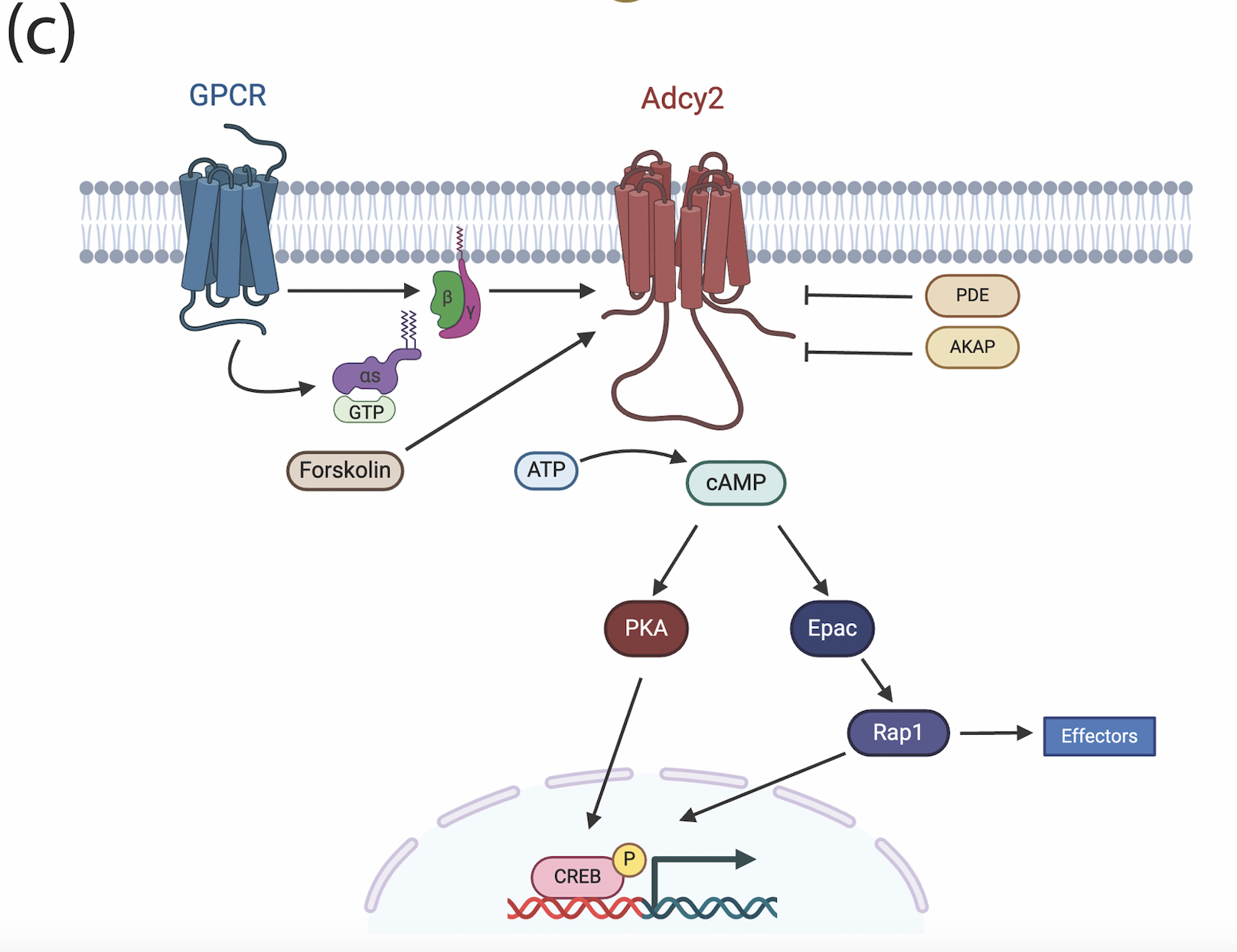 Adenylyl cyclase 2 (Adcy2) is a membrane-bound enzyme that catalyzes the formation of cAMP, a critical intracellular signaling molecule. RNAseq analysis has identified Adcy2 as negatively regulated by endothelial cell-derived laminin, which is critical for the formation and integrity of the blood-brain barrier (BBB). We are currently investigating the role of Adcy2 in homeostatic conditions as well as in cerebrovascular diseases that disrupt the BBB, such as hemorrhagic stroke. To do this we have generated novel mouse lines targeting Adcy2 to enable in vivo studies.
Adenylyl cyclase 2 (Adcy2) is a membrane-bound enzyme that catalyzes the formation of cAMP, a critical intracellular signaling molecule. RNAseq analysis has identified Adcy2 as negatively regulated by endothelial cell-derived laminin, which is critical for the formation and integrity of the blood-brain barrier (BBB). We are currently investigating the role of Adcy2 in homeostatic conditions as well as in cerebrovascular diseases that disrupt the BBB, such as hemorrhagic stroke. To do this we have generated novel mouse lines targeting Adcy2 to enable in vivo studies.
